Как выбрать гостиницу для кошек
14 декабря, 2021
Adsorption constitutes a solid sorption process by which the binding forces between fluid molecules (adsorbate) and the solid medium (adsorbent) derive from an electrostatic origin or from dispersion — repulsion forces. It is an exothermic process as a result of the gas-liquid phase change. The energy liberated during adsorption is called isosteric heat, and it depends on the nature of the adsorbent — adsorbate pair. The theory adopted is the Dubinin-Polanyi theory of volume filling of micropores [2], where the isosteric heat of adsorption can be given as
1.1 Ideal thermodynamic cycle
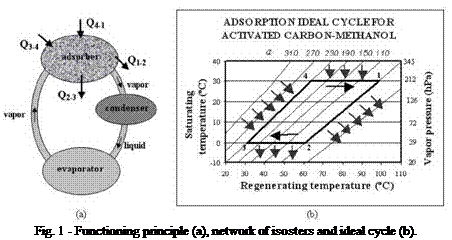
The adsorption refrigeration cycle consists of two well-defined stages: one is described as the adsorber cooling, with its consequent adsorption process, when the evaporation of the working fluid (the adsorbate) takes place. The other stage consists of the solid medium (the adsorbent) regeneration, when the adsorbate is desorbed and condensed (Fig. 1a). The ideal thermodynamic cycle can be represented by two isosters (isolines with constant adsorbed phase concentration, a) and two intercalated isobars, as shown in Fig. 1b.
The adsorber cooling corresponds to the isosteric process 1-2, depending on the ambient conditions. This process continues until the adsorber pressure reaches its minimum value (point 2), when it becomes equal to the evaporator pressure. At this point, the adsorption process starts and prolongs until its temperature reaches the minimum value (point 3). Then, the adsorber is heated, corresponding to
another isosteric process (3-4), until its pressure reaches a maximum value (point 4). Desorption starts at this point, and goes on until the adsorber temperature reaches its maximum value (point 1), completing, in this way, the cycle.