Как выбрать гостиницу для кошек
14 декабря, 2021
Figure shows a summary of the work done to produce selective paint. It is possible to observe high solar absorption values, but undesirably also high thermal emissivity. The paints obtained until the moment are not selective.
In the initial work with paints, the objective was to get good optical properties for paint with the organic pigment C6o/ C70. High solar absorption (95% and 96%) was reached. The problem was the emissivity, which is strongly dependent on coating thickness. With the coil method adopted for coating, the lower thickness achieve was 7pm, with 80% of emissivity and 95% of solar absorption. To reduce the thickness and consequently the emissivity, spray technique was tested and it was possible to achieve 4pm of thickness and emissivity of 74%, with 96% absorption.
|
Fig. 6. Absorption variation with wavelength for different paint samples. |
Without the possibility to reduce thickness to lower values with methods of easy application, it was also tested the incorporation of metallic pigments in the paint with 16% CVP of C60/ C70 pigment, considering that the thermal conductivity of metallic pigments would lower the emissivity values. Both copper pigment with average grain size between 63 and 90pm and stainless steel with average grain size of 3 pm were tested. The mix was done adding 16% of metallic pigment weight to the already prepared paint with C60/ C70 pigment. Figure 6 shows that this did not improve the paint behaviour in relation to emissivity.
Adding higher quantity of metallic pigment, about 50%, to the base of paint, without use of organic pigments, hoping to increment thermal conductivity of the coating and obtain lower emissivity,
independently of thickness coating, also did not improve the emissivity and, without organic pigment, the absorption decreased to 36%. The fact that the metallic pigment used, was stored for a long time (surface highly oxidized) could cause the observed behaviour. Also the surface shape of used pigments could explain the observed behaviour, since the surface contact area between metallic particles and the metallic substrate was not adequate to increment conductivity. These aspects will be explored in near future.
Topography of paint with organic pigment obtained by SEM (Fig.7.a) allows us to identify a granular morphology, with grains agglutinated by resin. It is visible agglomerates of small grains; which rough surface that can improve absorption.
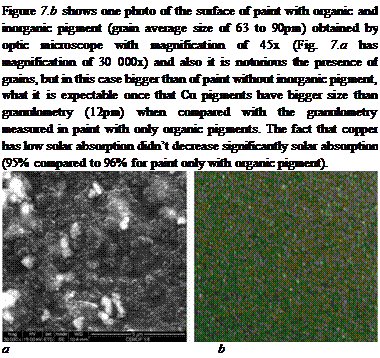
Fig. 7. a) SEM (30000x) surface micrograph of paint with organic pigment. b) Surface
photography by optic microscope (45x) of paint with organic and Cu grains.
Optical properties of titanium oxide are strongly dependents of deposition parameters, and some of these are interrelated, which become very difficult to relate optical properties with change of each parameter, but it is possible to conclude that best values of absorber selectivity were obtained in dc mode and in pulsed dc mode with 200kHz, with oxygen flow rate changing between 0 and 2.5ml/min with adequate slope. Adequate slope depends of deposition rate which depends of deposition power, total pressure, oxygen partial pressure and pulsed frequency and all of these parameters are important, once that for solar absorber selectivity the final thickness and oxygen gradient concentration along of the film thickness are determinants. Best optical properties for oxide titanium sputtered films were 88% for solar absorption, with 7% of emissivity for deposition parameters of: pulsed frequency 200kHz, reverse time of 0.4ps, discharge current of 0.7A, argon flow rate of 50ml/min and oxygen flow rate changing from 0 to 2.5ml/min. The morphology of oxide titanium films is columnar, with columns oriented in direction of growing film, which seem to be continuous from the substrate to the top of the film. Subsequent immersion in solution with antocyanin didn’t show to improve solar absorption.
For paints, the results obtained until the moment weren’t satisfactory. The best couple values for solar absorption and emissivity were respectively 94%, and 74%. Emissivity is dependent on thickness of coatings and with the used application techniques, the minimum thickness reached was 4pm, not low enough to obtain infrared transparency. The effort to reduce emissivity of paints adding metallic particles were unfruitful, at least using for the shapes and sizes of metallic particles used. Surface topography shows grains agglutinated with binder.
Aknowledgements -To Fundagao para a Ciencia e Technologia by the financial support through the
referred research project POCTI/ENR/62660/2004 “Development of new spectrally selective coatings with
organic pigments for absorbers of solar collectors.”
[1] Project POCTI/ENR/62660/2004 “Development of new spectrally selective coatings with organic pigments for absorbers of solar collectors”, Fundagao para a Ciencia e Tecnologia.
[2] M. J. Brites, C. Nunes, S. Vieira, D. Quintino, Lopes Prates, J. Alexandre, V. Teixeira, M. J. Carvalho, Proceedings of CIES 2006-XIII Iberic Congress, and VIII Iber American Congress of Solar Energy, Lisboa, Portugal, 9-10 November 2006.
[3] A. J. Martins, C. Nunes, M. J. Brites, M. Lopes Prates, V. Teixeira, M. J. Carvalho, Journal of Nanoscience an Nanotechnology, Vol. 8, 1-5, 2008.
[4] C. Nunes, V. T eixeira, M. L.Prates, N. P.Barradas, and A. D. Sequeira, Thin Solid Films 442, 173 (2003).
[5] M. J.Brites, C. Santos, B. Gigante, S. Nascimento, H. Luftmann, A. Fedoro v, and M. Berberan-Santos, New J. Chem. 30, 969 (2006).
[6] S. Nascimento, M. J.Brites, C. Santos, B. Gigante, A. Fedoro v, and M. Berberan-Santos, J. Fluoresc. 16,
245 (2006).
[7] K. Hara, Y. T achibana, Y. Ohga, A. Shinpo, S. Suga, K. Sayama, H. Sugihara, and H. Arakawa, Sol. Energy Mater. and Sol. Cells 77, 89 (2003).
[8] M. K.Gunde, Z. C.Orel, and M. G.Hutchins, Sol. Energy Mater Sol. Cells 80, 239 (2003).
[9] Z. C.Orel, Sol. Energy Mater. Sol. Cells 68, 131 (2000).
[10] Z. C.Orel, Sol. Energy Mater. and Sol. Cells 68, 337 (2001).
[11] K. Zakrzewska, M. Radecka, A. Kruk, W. Osuch, Solid State Ionics,8404 (2002).
[12] S. G.Springer, PE. Schmid, R. Sanjines, F. Levy, Surface and Coating Technology 151-152 (2002) 51-54.
[13] J. Y. Kim, EBarnat, E. J. Rymaszewski, T. M. Lu, J. Vac. Sci. Technology A 19(2), 429-434, Mar/Apr 2001.
[14] V. Vancoppenolle, P. Y.Juan, M. Wautelet, J. P.Douchot, M. Hecq, Surface and coating technology 116119 (1999) 933-937.
[15] S. G.Springer, PE. Schmid, R. Sanjines, F. Levy, Surface and Coating Technology 151-152 (2002) 51-54.
[16] P. S. Henderson, P. J.Kelly, R. D.Arnell, H. Backer, J. W. Bradley, Surface and Coating Technology 174175 (2003) 779-783.
[17] R. D. Arnell, P. J. Kelly, J. W. Bradley, Surface and Coating Technology 188-189 (2004) 158-163.
[18] N. J. Cherepy, G. P. Smestad, M. Gratzel, J. Z. Zhang, J. Phys. Chem B 1997, 101, 9342-9351.
[19] H. Backer, P. S. Henderson, J. W.Bradley, P. J.Kelly, Surface and Coating Technology 174-175 (2003) 909-913.
[20] A. Belking, Z. Zhao, D. Carter, L. Mahoney, G. McDonough, G. Roche, R. Scholl, H. Walde, Society of Vacuum Coaters, 43rd Annual Techn. Conf. Proc.-Denver, April 15-20, 2000.
[21] Jindrich Musil, Jan Lestina, Jaroslav Vlcek, Tomas Tolg, J. Vac. Sci. Technology A 19(2), 420-424, Mar/Apr 2001.
[22] I. Safi, Surface and Coatings Technology, 127 (2000) 203-219.
[23] P. J. Kelly, C. F. Beevers, P. S. Henderson, R. D. Arnell, J. W. Bradley, H. Backer, Surface and Coating Technology, 174-175, 795-800, 2003.
[24] Sang-Wong Park, Jae-Eun Heo, Separation and Purification Technology 58 (2007) 200-205.