Как выбрать гостиницу для кошек
14 декабря, 2021
The principle setup of membrane distillation (MD) is based on a hydrophobic, microporous membrane as shown in figure 1. Due to the high surface tension of the polymeric membrane materials, liquid water is retained from entering the pores while molecular water in the vapor phase can pass through.
A hydrophobic membrane is characterized by the fact that the surrounding liquid water can not enter its pores (capillary depression). This effect depends on the relative intensity of cohesiveness between the liquid molecules and the adhesive power between liquid molecules and the membrane material. These forces are responsible for the contact angle © = 180 — © between the liquid surface and the membrane wall. In case of a non wet-able membrane, the contact angle is © > 90° and a convex meniscus as shown on the right hand side of figure 1 is formed. The hydrostatic pressure of the water columns on both sides of the membrane must remain less than the wetting pressure of the membrane in order to restrict liquid water from passing the pores.
Temperature and vapour pressure
profile across the membrane
Evaporator Condenser
channel channel
Fig 1: Principle of direct contact membrane distillation (DCMD)
Membranes for MD usually have a pore diameter of 0.1- 0.4 pm and are made from PTFE, PVDF or PP polymers. The driving force in a MD process is the vapor pressure difference across both
membrane interfaces. For direct contact membrane distillation (DCMD) , this vapor pressure difference arises due to a temperature gradient between a hot feed stream in the evaporator channel and a cooled permeate stream in the permeate channel.
In MD, it is assumed that mass transfer is based on convection and diffusion of water vapor through the microporous membrane. Finally, summarizing the constants of different equations describing the convection and diffusion process in a single transport coefficient C leads to the simplified equation: Nw = C ■ dp
Usually C is determined by experimental investigations and is in the order of 3■ 10-7 to 4■Ю6kg/m2sPa depending on membrane material and geometry.
As can be seen in the graph of figure 2, the pressure difference across the membrane dp is calculated for the temperature differences between the hot and cold membrane interface according
figure 1 AT10 = T1 — T0 from the gradient of vapor pressure curve dP at average temperature, dT
T = 0.5 ■ (T1 + T0). Respectively the mass transfer can be calculated according the following
 Nw = СІР (71- T.)
Nw = СІР (71- T.)
dT
The gradient can be calculated using the Clausius-Clapeyron equation with sufficient
dT
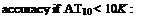 dp Ahv
dp Ahv
dT RT2
Where Ahv is the latent heat needed for evaporation.
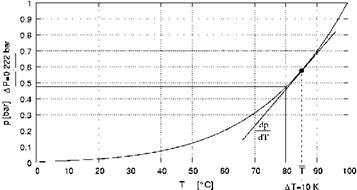
Fig 2: Pressure difference across the membrane dp calculated for the temperature differences dT between the
hot and cold membrane interface
There are three steps of heat transfer in MD. The first is the heat transfer from the hot bulk stream in the evaporator channel to the membrane interface. It is calculated as a function of the heat transfer coefficient a1 and the temperature difference between the bulk stream and membrane
interface Th — Tx. The second step is the heat transfer through the membrane. It consists of three different mechanisms: first is the heat transfer of the latent heat of vaporization Ahv which is transported with the vapor flux Nw through the membrane. The second mechanism is the heat flux through membrane material and the third is the heat conduction through water vapor and air in the membrane pores. Heat conduction through the membrane is adversarial because this fraction of energy can not be utilized for evaporation and must be considered as heat loss.
The third step is the heat transfer from the cold membrane interface to the cold bulk stream in the condenser channel.
Today MD is not used in large scale desalination plants. There are, however, several advantages which make this a preferred technology for small plants especially in remote applications where low temperature waste heat or a solar thermal heat supply is available, facilitating the energy selfsufficient operation of the unit.
The main advantages of MD are:
• The operating temperature of the MD process is in the range of 60 to 80 °C. This is a temperature range where solar thermal flat plate collectors have a sufficient efficiency or waste heat from cogeneration plants is available.
• The membranes used in MD are resistant against fouling and scaling.
• Chemical pre-treatment of the water supply is not necessary.
• Intermittent operation of the module is possible without heat storage.
• ![]() The system efficiency and the produced water quality are almost independent from the the raw water.
The system efficiency and the produced water quality are almost independent from the the raw water.