Как выбрать гостиницу для кошек
14 декабря, 2021
A. Hauer
Bavarian Center for Applied Energy Research — ZAE Bayem Dept. 1: Technology for Energy Systems and Renewable Energy Walther-Meissner-Str. 6, D-85748 Garching hauer@muc. zae-bayern. de
Abstract
Based on the laws of thermodynamics and the sorption theories, possibilities and limits of sorption storages for solar applications can be defined. For the storage of thermal energy closed and open sorption systems as well as solid and liquids sorbent materials can be utilized. Each system has its own advantages and disadvantages. Over the last years a number of R&D activities were performed. Examples are a closed adsorption storage system in Austria for seasonal storage of solar heat and an open liquid absorption storage system for solar cooling in Singapore. The conclusion of this paper is that even the high storage capacities and the possibility of providing heat and cold of sorption storages do not solve all solar thermal storage problems. It is still necessary for each system constellation to find an appropriate application and to carefully check the relevant boundary conditions.
Keywords: Sorption Storage, Adsorption, Absorption, Liquid Desiccant Cooling
Thermal Energy Storage (TES) is crucial for the efficient use of solar energy. TES systems are able to buffer the variable supply of solar radiation in a short term TES. Seasonal TES systems are able to keep the thermal energy surplus from summer to winter.
A high storage capacity and low thermal losses over the storage period are preferable for TES systems in general. Looking at the three possible technologies for TES — sensible, latent and thermochemical TES — thermochemical systems seem to have optimal properties for solar thermal applications, since they are able to achieve the highest capacities and have no thermal losses.
Thermochemical TES are utilizing reversible chemical reactions. The number of possible reactions for this application from first principle is huge, however only very few are suitable with respect to their reaction temperature. The processes of adsorption on solid materials or absorption on liquids are the most investigated ones. Figure 1 shows the adsorption process schematically.
|
Adsorption means the binding of a gaseous or liquid phase of a chemical component on the inner surface of a porous material. During the desorption step — the energetic charging step — heat is provided to the sample. The adsorbed components — in this example water molecules — are removed from the inner surface. As soon as the reverse reaction — the adsorption — is started by adding water molecules to the sample, the molecules will be adsorbed and the heat, brought into the system during desorption will be released. The adsorption step represents the discharging process.
Figure 2 shows the examples of liquid and solid open sorption storage systems. In both cases the desorption is activated by a hot air stream carrying the heat of desorption. For the solid a packed bed of adsorbent pellets and for the liquid solution a reactor are blown through, leaving the packed bed dry and the solution concentrated.
|
air + water :nt ed air |
|
Adsorbent Packed Bed! |
|
air + water |
|
Absorber |
|
heat of |
|
S ДЩД ~~ -| Ї- |
|
oncentrated Salt Solution |
|
Diluted |
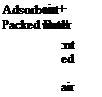 |
 |
Solid Adsorbent Liquid Absorbent
Figure 2: Examples of open sorption storage systems during desorption / charging
TES can be achieved by separating the desorption step (charging mode) from the adsorption step (discharging mode). After desorption the adsorbent and the absorbent can theoretically remain in the charged state without any thermal losses due to the storage period until the adsorption process is activated.
|
Humidifiei |
|
Cool |
![]()
![]() Solid Adsorbent Hot
Solid Adsorbent Hot
і і 0гУ
Liquid Absorbent Dry Cool
Figure 3: Examples of open sorption storage systems during adsorption / discharging
Figure 3 shows schematically the discharging of open sorption storages. Humid air blown through the storage becomes dry and can be used for dehumidification or, by adding a
humidification step, for cooling (desiccant cooling systems). If solid adsorbents are used the air might be very hot after the adsorption. This heat can be used for heating purposes.
Open and Closed Sorption Storages
The charging process of a sorption TES is a reaction where two components A and B — adsorbent and adsorbed water — will be separated by the input of heat Q and entropy S : AB o A + Bg. The water will be evaporated in this step.
In an open system Bg can be released into the ambience (see figure 4). For the discharging Bg has to be provided to the reactor in sufficiently high concentrations. The water vapour will be adsorbed again and the stored heat can be released.
Figure 5: Schematic view of the thermodynamics of a closed sorption storage
The evaporated component Bg will be condensed during the charging step in a closed (evacuated) system. This is in order to reduce the volume. The heat of condensation and its entropy have to be dissipated into the ambience. For discharging the condensed water has to be evaporated again in order to be adsorbed at the reactor [1].
Closed Sorption Storage Systems
A closed sorption system is shown in figure 6. It is based on the same physical effect as the open storage. However the engineering is quiet different from open sorption systems. Closed system could be more precisely described as evacuated or air-free systems. The operation pressure of the fluid to be sorbed can be adjusted in theses systems. In closed systems chemical components, which do not exist in the atmosphere, can be used, because there is no connection to the ambience.
Figure 6 is showing a closed sorption system using water vapor as adsorptiv. The heat has to be transferred to and from the adsorbent by a heat exchanger. This holds also for the condenser/evaporator. Heat has to be transported to the adsorber and at the same time the heat of condensation has to be distracted from the condenser in order to keep up the water vapor flow from the adsorber to the condenser during the desorption. During adsorption the heat of adsorption has to be taken from the adsorber and the heat of evaporation has to be delivered to the evaporator. Is
this not possible, the sorption process will reach thermodynamic equilibrium and the flow of water vapor comes to a stop.
The main problem in the system design is the heat and vapor transport in and out of the adsorbent. Advanced heat exchanger technologies have to be implemented in order to keep up the high energy density in the storage, which would be reduced by the amount of "inactive" heat exchanger material.
|
Water Vapor Water Vapor
QDes Qcond QAds QEvap Figure 6: Closed Sorption system |
Thermal energy storage can be realized by closing the valve between adsorber and condenser/evaporator after desorption. The energy density expected is reduced compared to open sorption storages due to the fact that the adsorptive (water vapor in this case) is part of the storage system and has to be stored as well. In the case of Zeolite or Silicagel as adsorbent this is about 30% to 40 % of the weight of the storage material [2], [3].
Closed systems are able to reach higher output temperatures for heating applications compared to open systems. Furthermore they can supply lower temperatures for cooling, e. g. it is possible to produce ice in the evaporator [4].