Как выбрать гостиницу для кошек
14 декабря, 2021
Fig. 2 shows inorganic materials which have been proposed as a PCM. For comparison also two metals are shown. The lightweight alkali metal lithium has a high melting enthalpy, whereas metals with a high molar mass, such as tin show low enthalpies (Fig. 2). One major disadvantage of lithium is the handling. Tin has a high thermal conductivity of a few 10 W/mK in the solid and liquid phase around the melting temperature. However, for large scale systems tin is not attractive from an economical point of few due to the low melting enthalpy and the high price per mass. For temperatures below 100°C, salt hydrates are a major PCM option. There are also salt hydrates above 120°C (Fig. 2)[7,24]. However, the release of water and encapsulation at these temperatures can be regarded as challenging.
|
Metals (solid-liquid) |
|
Anhydrous Salts |
|
Alkali Lithium 181°C, 432 J/g |
|
Others Classified by anions, typical cations are Li, K, Na, Ca, Mg |
|
solid-liquid Nitrates Nitrites Hydroxides Thiocyanates Chlorates Chlorides |
|
solid-solid Na2SO4 250°C, 60 J/g KOH 250°C, 110 J/g |
|
Salt Hydrates MgNO32H2O 130°C MgBr2-6H2O 172°C |
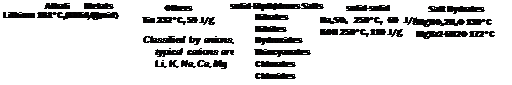 |
— Inorganics
Fig. 2. Inorganic PCMs in the temperature range 120 to 250°C.
There are solid-solid and solid-liquid phase transitions of anhydrous salts which can be utilized. As low cost PCM with solid-solid phase transition, sodium sulphate (Na2SO4) has been examined [24, 25]. The transition enthalpy is rather low (Fig. 2) and difficulties with the mechanical stability
have been reported [25]. Other solid-solid PCMs, such as anhydrous KOH, have been also suggested (Fig. 2)[1,5,24].
There are different types of anhydrous salts and salt mixtures with a melting temperature in the range 120 to 250°C. Table 2 summarizes suggested PCMs in thermal energy storage literature classified by their anions [1,3,5,7,8,9,24,26-30]. Additionally, there may be other salts with a suitable melting temperature, such as alkali metal thiocyanates.
|
Table 2. Suggested anhydrous salts and binary mixtures with a melting temperature from 120 to 250°C.
|