Как выбрать гостиницу для кошек
14 декабря, 2021
One way to improve storage is to use a material that can be more energy dense than water. If the transition temperature is adequately chosen, such materials exist. Task 32 worked with sodium acetate which exhibits a theoretical transition point at 58 C, ideally suited to solar combisystems.
Material caracterisation is a topic in itself and Task 32 developped a method to be able to assess the thermal properties of the material in a confident and repeatable way (figure 4).
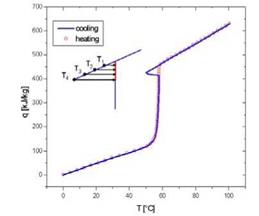 Fig. 4. Thermal properties of sodium acetate trihydrate with graphite, measured at IWT, Austria. The material supercooling effect is clearly visible. Hysteresis must also be precisely measured.
Fig. 4. Thermal properties of sodium acetate trihydrate with graphite, measured at IWT, Austria. The material supercooling effect is clearly visible. Hysteresis must also be precisely measured.
Modelling PCM in a storage is also a difficult item that has been adressed by Task 32. We have now models of sodium acetate in different configurations that can be used to predict the behaviour of solar combisystems with such PCM storages.
Simulation of several combisystems with PCM storage revealed that PCM storage show two unforeseen limitations: the volume of PCM that one can introduce into a water tank is limited to 20 to 30% of the whole storage volume, due to the necessary containment of the material and the heat extraction power from PCM to water is limited by the diffusion process inside the material. Sodium acetate did not show a decisive advantage over water in a combitank even if its density of energy is higher and if its heat conductivity is enhanced with graphite. Special system design were developped which showed better improvements but with a 35 C transition temperature material.
Fine tuning of the adequate PCM and the proper way to build an efficient heat exchange should remain on the agenda of international solar research. A new idea is being investigated in Denmark: using the supercooling effect of sodium acetate to build a seasonal store with limited heat losses.