Как выбрать гостиницу для кошек
14 декабря, 2021
The x-rays emissions with energy characteristics of the composite chemical elements let completed of the qualitative and quantitative elemental analysis on elemental compositions distributed on a sample surface and in a depth of 1-2 microns.
|
Fig.3. Point spectrum (yellow) of the point area A of the NaNO3 /KNO3 eutectics/graphite NG (20%) composite, compared to the integral spectrum (red) scanned for the sample section. Peak energy levels characterize chemical elements: K, Na, N2, O2, and C. |
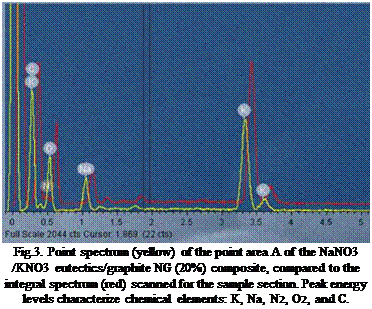 |
The EDS spectra were used for a qualitative element analysis of the composite sample. Peak energy levels characterize chemical elements: K, Na, N2, O2, and C. (Fig.3.). Comparable EDS qualitative elemental analysis on both, a point elemental spectrum and an integral elemental spectrum made on the Area A of NaNO3 /KNO3 eutectics/ graphite NG (20%) composite indicate negligible shifting in energy levels.
Different areas and segments of the same composite sample were EDS scanned, but the element determination shows variety of elemental distribution in different sections taken across the solidified salt melts in the graphite matrix. The element concentrations in micro-volumes of the composite were calculated by quantitative element analysis of the peak energy levels from EDS spectra. The calculated data for element concentration of two different area of the same sample were summarized in Table 5.
|
Table 5. EDS quantitative point elemental analysis of the NaNO3/KNO3eutectics/graphite (20%) composite. Element concentration is measured in weight percentage (wt%).
|
Element concentration presented on Table 5, suggest the element K-rich area in the point area A of the composite sample scanned. The concentration of the element K (from KNO3) is 21.69 wt%, calculated in the fixed point area A of a salt/graphite composite, while the concentration of the
element Na (from NaNO3) is calculated as 9,81 wt%. The calculated element concentrations at a point area B show different values than values for point area A. So, the chemical element concentrations vary depending on spectral area scanned.
The EDS calculated data illustrated clearly the variations in the K and Na element concentrations fixed at different point areas but scanned for the same sample of the salt/graphite composite after its re-crystallization. Therefore, the elemental distribution of salt eutectics is not homogeneous and defines some irregularity in the composite structure and thermophysical properties of the salt/graphite composite.
Thermal behaviour of the novel KNO3/NaNO3eutectics/graphite composite depends on intermolecular interaction of both components: salt and graphite. Correlation between thermal and structural analysis of the composite allows identifying the factors for efficient release of heat absorbed and stored.
Solidification of the re-melted KNO3/NaNO3 eutectics in graphite composite led to elemental separation and forming the Na-rich spherical masses and the K-rich layered structure over the graphite plane, clearly proved by methods of x-ray microanalysis. As the products of chemical degradation or chemical reaction were not identified, the inhomogeneous elemental distribution characterizes the salt in salt/graphite composite structure and presents a factor for proper eutectics salt crystallization, finally responsible for structural and thermal stability of composites during repeatable heat charging/discharging process.
[1] K. Lafdi, O. Mesalhy, A. Elgafy, “Graphite foams infiltrated with phase change materials as alternative materials for space and terrestrial thermal energy storage applications”, CARBON, 46 (2008) 156-167.
[2] Z. Zhang, X. Fang, “Study on paraffin/expanded graphite composite phase change thermal energy storage material”, Energy Conversion and Management, 47 (2006) 303-310.
[3] S. Pincemin, R. Olives, X. Py, M. Christ, “Highly conductive composites made of phase change materials and graphite for thermal storage”, Sol. En. Mat. & Sol. Cells, 92 (2008) 603-613.