Как выбрать гостиницу для кошек
14 декабря, 2021
Stephanie HONGOIS1,2, Philippe STEVENS*3, Anne-Sophie COINCE1, Frederic KUZNIK2,
* author for correspondence
Email : philippe. stevens@edf. fr
1 EDF R&D, Department ENERBAT, Avenue des Renardieres, 77818 Moret sur Loing Cedex, FRANCE
2 CETHIL, Thermal Sciences Center of Lyon, UMR 5008, CNRS, INSA-Lyon, Universite Lyon 1, Bat. Sadi Carnot, 20 rue de la Physique, 69100 Villeurbanne, France
3 EDF R&D, Department LME
A seasonal chemical heat store, based on the hydration/dehydration cycle of a magnesium sulphate composite material, has been developed. During the summer, the material stores heat by an endothermic dehydration reaction and heat used for space heating is released in winter by rehydrating the material. The first elements for the sizing of the heat storage material tank have been calculated by modelling a conventional solar combisystem. For a low energy house of 191 m2 located in Paris, with a heat demand of 37.2 kWh. m-2 for space heating (53.0 kWh. m-2 including domestic hot water), the volume of magnesium sulphate required to reach a solar fraction ranging from 50 to 57 % varies from 0.2 to 0.9 m3. For the same house located in Marseille, with a space heating demand of 15.4 kWh. m-2 (31.1 kWh. m-2 including domestic hot water), a 100% solar fraction is achievable with a volume of 0.7 m3 of magnesium sulphate.
The hydration-dehydration reaction occurs at a gas-solid interface. In order to optimise the rate of reaction and the thermal power released or absorbed, the active material needs to be dispersed, which increases the volume of storage to an estimated 2 m3 to reach 50 % of the theoretical energy density of the dense salt. Composite materials made of magnesium sulphate are being developed at EDF R&D with the aim of reaching an energy density of 150-400 kWh. m-3 at a storage temperature compatible with solar thermal panels. The hydration of MgSO4/zeolite composites in an open system leads to higher temperature lifts and usable heat than MgSO4/silica gel composites.
Keywords: thermal energy storage, thermochemical storage, magnesium sulphate.
Most of the final energy consumed in France is used in the form of heat (85 Mtoe over a total of 161 Mtoe in 2004). Heat is also the largest part of the final energy used in buildings (around 46% of the total energy) and is responsible for 25% of the greenhouse gas emissions. It has therefore a considerable potential for energy savings and is a key element in the reduction by a factor of four of the GHG emissions by 2050 in Europe [1]. Coupled with actions to reduce existing dwellings heat demand through thermal insulation and better energy management, the use of high efficiency systems and renewable energy sources is necessary to fulfil this goal.
The available solar energy is huge, but the main difficulties lie in the diurnal and seasonal variations of the resource. Solar heat is available in excess in summer months, whereas a deficit occurs during the winter months (Fig. 1).
kWh / month
|
1 : Wasted energy 2 : Heat demand covered by solar energy 3 : Heat demand covered by auxiliary heating |

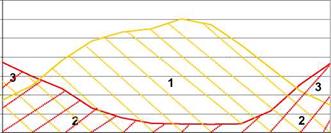 3500
3500
_ 3000 .c
I 2500 E
^ 2000 £
1500
>
S 1000 c Ш
500
0
— Total heat demand of the building — Solar irradiation
Fig. 1. Monthly plot of total thermal energy demand vs. solar radiation on the collectors — House located in Paris
To overcome seasonal variations, a long-term storage should be designed. Thermal energy can be stored using one of three principles :
• sensible heat storage, based on a change in temperature of a material such as water, with a thermal energy density of around 70 kWh. m-3
• latent heat storage, based on the energy required or released during a phase change of a solid or liquid, with thermal energy densities between 40-100 kWh. m-3
• thermochemical heat storage, based on a sorption or a chemical reaction, with thermal energy densities between 150-1500 kWh. m-3.
The storage of heat using a chemical reaction is the most suited to seasonal heat storage because of the lack of thermal losses. Moreover, its volumetric energy density can be much greater than other forms of storage. During the summer, the material stores heat by an endothermic dehydration reaction whereas heat is released by rehydration in winter and used for space heating. Composite materials are being developed at EDF R&D with the aim of achieving usable energy densities between 150-400 kWh. m-3 at a storage (dehydration) temperature compatible with solar thermal panels.