Как выбрать гостиницу для кошек
14 декабря, 2021
1.2. Energy and Exergy
The operation of a typical TES can be separated into three key segments: charging, discharging, and storage. The energy balance within the TES adheres to First Law conservation of energy, such that:
tit t
|
(1) |
![]()
|
0 |
|
0 |
|
0 |
|
0 |
f Estored)dt = f Echarged(t)dt “ f ^schargedW^ “ f Elost(t)dt
|
riER (t) = |
|
E |
|
stored, real |
|
E. |
|
stored, ideal |
|
(0 (t) |
|
(2) |
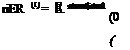 |
|
Elost(t) denotes the thermal energy lost to the surroundings. We otherwise ignore kinetic and potential energy effects. Early techniques for assessing thermal storage effectiveness consisted principally of First Law considerations [6], where the Energy Response (pER) of an experimental TES would be typically compared to an ideal system [1], such that:
|
£ stored (t) _ mstoredCp (^stored (t) T0 ) mstoredCpT0 ln |
|
T A |
It has been widely accepted that the energy response alone (a representation the First Law of Thermodynamics) cannot solely characterize a TES. Stratification, for instance, which is known to significantly improve the effectiveness of a thermal energy store [7], cannot be assessed using the First Law as it is a characteristic that describes how energy is stored in the tank, not the energy content itself. To incorporate stratification into a characterization scheme therefore requires the Second Law of Thermodynamics, which states that the optimal storage of useful energy in a TES is achieved when maximing the exergy stored [5], represented as:
T0 refers to the dead-state temperature at which work is to be performed. A review of TES characterization methods by Haller et. al. [8] further illustrates that the incorporation of Second Law figures of merit can improve the overall assessment of TES storage efficacy. The methods by Panthalookaran [1], Shah and Furbo [2], and Huhn (referenced in [8]) are particularly highlighted.