Как выбрать гостиницу для кошек
14 декабря, 2021
The hydration reaction involves a reaction between water vapor and the dehydrated material. This reaction determines the amount of power that can be delivered to a load in a practical system and the temperature level at which the power can be delivered.
|
Fig. 5. The effect of water vapor pressure (PH2O) and temperature (T) on hydration |
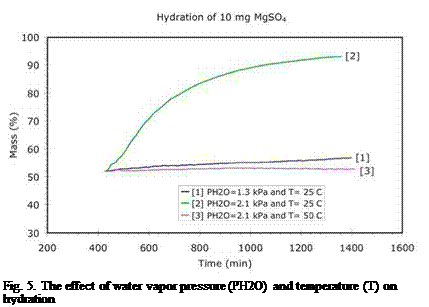 |
The first hydration experiments [8] at ECN indicated that the material was able to take up water after dehydration. Furthermore it was discovered that the layer thickness is an important factor in hydration: thick layers reduce the hydration rate considerably, indicating that the water vapor transport through the layer is the limiting factor. The experiments also suggested that the water uptake was strongly reduced when the hydration was performed at elevated temperatures and reduced water vapor pressures. To further investigate this observation, it was decided to study the effect of water vapor pressure and temperature on the hydration process for constant layer thickness. The results of these experiments are shown Figure 5:
First, the hydration was studied at 25°C and for a water vapor pressure corresponding to the practical conditions (PH2O=1.3 kPa), where a borehole at ground temperature provides the water vapor. In this case, the material slowly takes up a small amount of water (nr.1 in Figure 5). Increasing the water vapor pressure (PH2O=2.1 kPa, nr.2 in Figure 5) at 25°C, results in a larger amount of water taken up by the material, although the hydration rate is still slow. Additional experiments reveal that this large uptake of water results in the release of ~91% of 2.3 GJ/m3 (or 2.1 GJ/m3) of the energy stored during dehydration. The results presented in Figure 5 show that the hydration strongly depends on the water vapor pressure and suggest that the water vapor pressure provided by the bore hole at ground temperature may not be sufficient to release all of the energy stored during dehydration.
The effect of temperature on hydration was also investigated. A typical low-temperature domestic heating system requires >40°C for space heating. For this reason it was decided to study the hydration at 50°C at a water vapor pressure of 2.1 kPa. The result (nr.3 in Figure 5) shows that, although the water vapor pressure is almost twice the value used in a practical system (PH2O=1.3 kPa), the material is unable to take up water (and deliver heat) at 50°C. This means that the application of magnesium sulfate as thermochemical storage material is quite problematic.
The characterization of magnesium sulfate revealed that the material can be dehydrated using solar thermal collectors (<150°C). Almost 10 times more energy compared to water of the same volume can be stored using magnesium sulfate. The hydration experiments revealed that the material is also able to take up water while releasing a part of the stored energy. However, the amount of energy released during hydration depends on the temperature and the water vapor pressure. The experimental results indicate that the material cannot release all the stored heat under practical conditions, which severely limits the application of magnesium sulfate as thermochemical storage material.
Nevertheless, the characterization of magnesium sulfate gives us much needed insight in the dehydration and hydration behavior of a salt hydrate. Although the application of magnesium sulfate as TCM turns out to be quite problematic, it gives a blue-print on how to characterize salt hydrates for thermochemical heat storage. It is expected that other salt hydrates can perform better, and the characterization of magnesium sulfate is a reference point for future characterization of other salt hydrates for thermochemical seasonal heat storage.
This research was performed within the WAELS project: a cooperation between ECN, TNO, and the Eindhoven University of Technology, and partly funded by SenterNovem, an agency of the Dutch Ministry of Economic Affairs. The work on thermochemical heat storage is part of the long-term work at ECN on compact storage technologies.
[1] Visscher, K. Veldhuis, J. B.J., Oonk, H. A.J., van Ekeren, P. J. and Blok, J. G.,. (2004) ECN Report ECN-C— 04-074: Compacte chemische seizoenopslag van zonnewarmte; Eindrapportage, ECN, Petten
[2] Emons, H. H., Ziegenbalf, G., Naumann, R. and Paulik, F., Thermal decomposition of the magnesium sulfate hydrates under quasi-isothermal and quasi-isobaric conditions, J. Therm. Anal. 36, 1265-1279 (1990)
[3] Ruiz-Agudo, E., Martin-Ramos, J. D., and Rodriguez-Navarro, C., Mechanism and kinetics of dehydration of epsomite crystals formed in the presence of organic additives, J. Phys. Chem. B. 111, 41-52 (2007)
[4] Hamad, S. El D., An experimental study of salt hydrate MgSO4.7H2O, Therm. Acta 13, 409-418 (1975)
[5] Phadnis, A. B, and Deshpande, V. V., On the dehydration of MgSO4.7H2O, Therm. Acta 43, 249-250 (1981)
[6] Paulik, J., Paulik, F., and Arnold, M., Dehydration of magnesium sulfate heptahydrate investigated by quasi isothermal-quasi isobaric TG, Therm. Acta 50, 105-110 (1981)
[7] Van der Voort, I. M., Characterization of a thermal chemical material storage, Eindhoven University of Technology, 2007
[8] Zondag, H., Essen, V. M. van, He, Z., Schuitema, R. and Helden, W. van, Characterization of MgSO4 for thermochemical storage, in Second International Renewable Energy Storage Conference (IRES II), Bonn, Germany (2007)